Definition Of Buffer In Pharmacy
Cleanroom Buffer Area Buffer or Core Room Buffer or Clean Room Areas Buffer Room Area Buffer or Clean Area or Buffer ZoneAn ISO Class 7 area where the primary engineering control area is physically located. BUFFERS IN PHARMACEUTICAL SYSTEMS Solid Dosage FormsBuffers have been widely in solid dosage forms such as tablets capsules and powders for controlling the PH of the environment around the solid particles.
 White Pipes With Yellow Stickers And Chrome Fixtures Make Them Look More Sterile Than Their Indus Pharmaceutical Manufacturing Pharma Companies Pharmaceutical
White Pipes With Yellow Stickers And Chrome Fixtures Make Them Look More Sterile Than Their Indus Pharmaceutical Manufacturing Pharma Companies Pharmaceutical
Buffer definition is - fellow man.

Definition of buffer in pharmacy. Buffer Room A type of C-SEC where the C-PEC is located. How to use buffer in a sentence. I am working as Assistant Professor in a Pharmacy College and trainer in Vibgyor Laboratories.
2 Ionization of Solutes. Coloring agents They are added to impart desired color to suspension and improve elegance. Microorganisms in the environment are monitored so that a microbial.
Buffers and pH adjusting agents They are added to stabilize the suspension to a desired pH range. The common spaces found in a working pharmacy include the general pharmacy anteroom and buffer rooms. 1116 and also the definition of.
Activities that occur in this area include the preparation and staging of components and supplies used when compounding sterile preparations. It is a unitless number. Buffers to regulate the pH of the formulation eg.
It may also contain. One of the special applications of buffers is to reduce the gastric irritation caused by the acidic drugs. Electrolytes Are solutes which dissociate into ions if the dielectric constant of the solvent is high enough to cause sufficient separation of the attractive forces between the oppositely charged ions.
Non-ISO classified negative pressure room with at least 12 air changes per hour. Osmotic agents They are added to adjust osmotic pressure comparable to biological fluid. Buffer solutions are solutions that resist changes in pH upon addition of small amounts of acid or base or upon dilution.
Buffers in Pharmacy 1. Hello Students I am Anurag Jaiswal. The walls floors ceilings and all surfaces must be smooth and easy to clean and only a minimal amount of furniture may remain in the room.
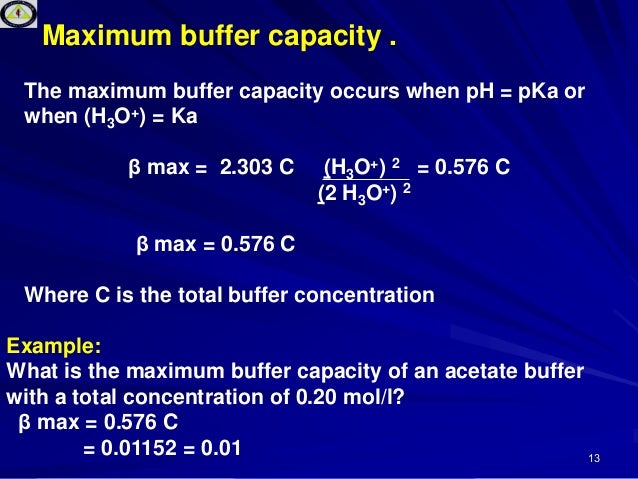
American Society of Heating Refrigerating and. Buffer capacity β is defined as the moles of an acid or base necessary to change the pH of a solution by 1 divided by the pH change and the volume of buffer in liters. Definition of a Buffer which you can easily find in every book or site is.
A buffer resists changes in pH due to the addition of an acid or base though consumption of the buffer. Isotonic Buffers The addition of any compound to a solution will affect the isotonicity since isotonicity is a property of the number of particles in solution. Chapter outlines minimal cleaning and disinfecting requirements for the typical Sterile Compounding Pharmacy environment.
So the osmotic pressure of a solution will be affected not only by the drug but also by any buffer compounds that are included in the formulation. A buffer has no role in a pharmacy store but definitely plays a significant role in the manufacturing of pharmaceutical products. Pharmaceutical Buffers Buffer solutions are used frequently in pharmaceutical practice particularly in the formulation of ophthalmic solutions.
Many buffers are available today. The following steps note the differences as they apply. Sometimes a storage room or HD storage room are included.
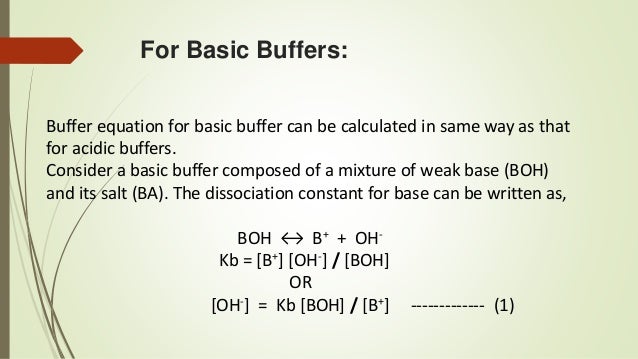
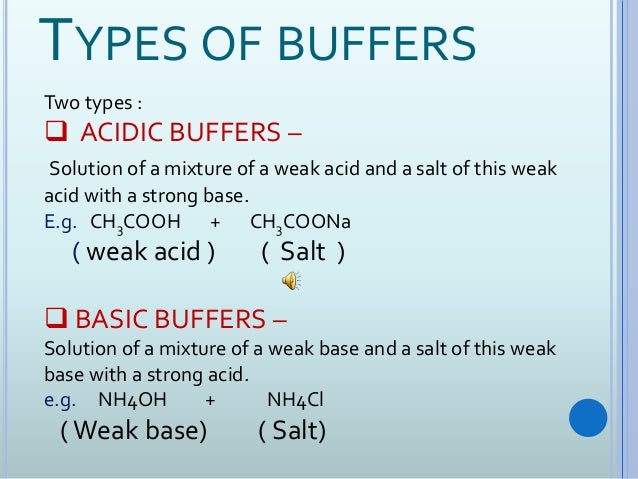
A mixture of an acid and its conjugate base salt such as H2CO3HCO3. About this video - Definition of Buffer Solution Types of Buffer. Examine the types and uses of pharmaceutical solutions as oral drug delivery systems.
Donning garb for HD sterile compounding differs depending on whether the pharmacy performs HD compounding in an HD buffer room ISO 7 negative pressure or in a containment segregated compounding area C-SCA. 1 chapter 1 Pharmaceutical solutions for oral administration In this chapter we will. From a pharmacological perspective it is important to control the pH of a solution to minimize drug degradation to improve patient comfort and compliance and to improve the efficacy of delivery.
Buffers are prepared in pharmacies or by drug manufacturers. The buffer room is the only absolutely sterile room in the pharmacy. H2PO4 HPO42- which when present in a solution resists changes in pH that would otherwise occur in the solution when acid or alkali is added to it.
The specific roles of each of these formulation excipients will be described later in this chapter. Buffer AreaA room in which the concentration of airborne particles is controlled to meet a specified airborne particulate cleanliness class. They adjust the pH of aqueous solutions for applications that require predictable stability and best clinical outcomes.
One of the most common biological buffers is phosphate buffered saline PBS. This has practical application for the drugs that have dissolution rate limited absorption from unbuffered solutions 1 2 3 4 5 6. Phosphate buffered saline contains sodium chloride NaCl and dibasic sodium phosphate Na2PO4.
Before understanding when these spaces are required and how they interact with one another it is important to understand a few basic definitions and terminology utilized in USP 800. Our recommendations are for pharmacies with a typical ISO Class 7 Buffer Zone however facilities with ISO Class 5 cleanrooms have more rigorous standards and may require different products than those listed here. In order for the pharmacy to remain up to code it must stay this way.
 Importance Of Buffer Solution In Pharmacy Pharmacywalls
Importance Of Buffer Solution In Pharmacy Pharmacywalls
 Buffer Solutions Math Methods Buffer Solution Mental Math
Buffer Solutions Math Methods Buffer Solution Mental Math
 Iv Fluids And Solutions Quick Reference Guide Cheat Sheet Nclex Quiz Iv Fluids Fluid And Electrolytes Iv Solutions
Iv Fluids And Solutions Quick Reference Guide Cheat Sheet Nclex Quiz Iv Fluids Fluid And Electrolytes Iv Solutions
What Is The Role Of A Buffer In A Pharmacy Quora
 Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
 Factor Affecting Uv Vis Absorption Lalit Reaction Rate Buffer Solution Molecules
Factor Affecting Uv Vis Absorption Lalit Reaction Rate Buffer Solution Molecules
 What Is Trehalose Teaching Chemistry Biology Class Biochemistry
What Is Trehalose Teaching Chemistry Biology Class Biochemistry
 Buffer Solution Types Buffer Capacity Inorganic Pharma Chemistry Youtube
Buffer Solution Types Buffer Capacity Inorganic Pharma Chemistry Youtube
 What Is Solubility Solubility Product Solubility 11th Chemistry Electron Configuration
What Is Solubility Solubility Product Solubility 11th Chemistry Electron Configuration
 Buffer Solution Its Characteristics Types And Preparations
Buffer Solution Its Characteristics Types And Preparations
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts
 Factor Affecting Uv Vis Absorption Lalit Buffer Solution Reaction Rate Molecular
Factor Affecting Uv Vis Absorption Lalit Buffer Solution Reaction Rate Molecular
 Chemical Structure Of Sucrose Teaching Chemistry Chemical Structure Biology Class
Chemical Structure Of Sucrose Teaching Chemistry Chemical Structure Biology Class